Background:
The CD20 molecule is universally expressed by normal B cells in all stages of development, from the pre-B cell up to the mature plasma cell as well as by most B cell malignancies including CLL, FL and BL (Chu/Cairo, BJH, 2016). Rituximab, a monoclonal chimeric anti-CD20 antibody, has been widely used as a chemoimmunotherapeutic regimen in the frontline therapy for patients with CD20+ BL and diffuse large B-cell lymphoma. The addition of rituximab to the CHOP backbone or to standard FAB/LMB therapy has greatly improved outcomes without significantly increasing toxicity in patients with B-NHL (Goldman/Cairo, Leukemia, 2013, Coiffier et al, NEJM, 2002). However, patients who relapse have a poor clinical response to rituximab retreatment. Obinutuzumab is a humanized, type II anti-CD20 monoclonal antibody glycoengineered to enhance Fc receptor affinity. It has lower complement-dependent cytotoxicity than rituximab but greater ADCC, phagocytosis and direct B-cell killing effects (Chu/Cairo, BJH, 2018). Obinutuzumab has been successfully utilized in front-line therapy in FLL (Marcus, et al, NEJM, 2017) and CLL (Goede, et al, NEJM, 2014; Moreno, et al, Lancet, 2019). Our group has successfully expanded functional and active peripheral blood NK cells PBNKwith irradiated feeder cells to target B-NHL (Chu/Cairo, et al, Can Imm Res 2015). We previously demonstrated that obinutuzumab has significantly enhanced expanded PBNK mediated cytotoxicity against BL and pre-B-ALL cell lines compared to rituximab (Tiwari/Cairo et al, BJH, 2015). NKTR-255 is an IL-15 receptor agonist designed to activate the IL-15 pathway and expand natural killer (NK) cells and promote the survival and expansion of memory CD8+ T cells without inducing suppressive regulatory T cells (Kuo/Zalevsky, Cancer Res. 2017). NKTR-255 stimulates proliferation and survival of NK, CD8+ T cells, and enhances long-term immunological memory which may lead to sustained anti-tumor immune response.
Objective:
To investigate the effects of NKTR-255 on the ADCC of expanded NK cells with anti-CD20 type I and type II antibodies against CLL, FL and rituximab-resistant BL.
Methods:
NK cells were expanded with lethally irradiated K562-mbIL21-41BBL cells as previously described (Denman/Dean Lee, PLoS One, 2012). Expanded PBNK cells were isolated using Miltenyi NK cell isolation kit. NKTR-255 was generously provided by Nektar Therapeutics. In vitro cytotoxicity was examined using luminescence reporter-based assays. IFNg, granzyme B and perforin levels were examined by standard enzyme-linked immunosorbent assays as we previously described (Chu/Cairo, ASH, 2018). MEC-1 (CLL), PGA-1 (CLL), DOHH2 (FL) and Rituximab-resistant BL cells Raji-2R and Raji-4RH were used as target cells.
Results:
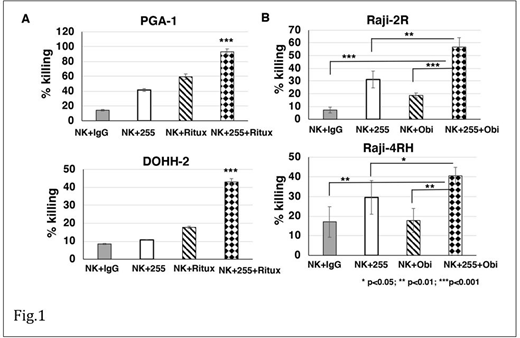
NKTR-255 significantly enhanced the in vitro cytotoxicity of expanded NK cells when combined with rituximab against MEC-1 (E:T=3:1, p<0.001), PGA-1 (E:T=3:1, p<0.001), and DOHH2 (E:T=3:1, p<0.001) as compared to the control groups (Fig.1A). NKTR-255 also significantly enhanced granzyme and perforin release from expanded NK cells when combined with rituximab against MEC-1 (granzyme: p<0.05; perforin: p<0.001), PGA-1(granzyme: p<0.05; perforin: p<0.05), DOHH2 (granzyme: p<0.05; perforin: p<0.001) as compared to controls.
NKTR-255 significantly enhanced the in vitro cytoxicity of expanded NK cells when combined with obinutuzumab agains rituximab-resistant BL cells like Raji-2R (E:T=3:1, p <0.01), and Raji-4RH (E:T=3:1, p<0.01) as compared to the control groups (Fig.1B). NKTR-255 also significantly enhanced IFN-g, granzyme and perforin release from expanded NK cells when combined with obinutuzumab against Raji-2R (E:T=3:1, IFN-g: p<0.001, granzyme: p<0.001 and perforin: p<0.001) and Raji-4RH (E:T=3:1, IFN-g: p<0.001, granzyme: p<0.01 and perforin: p<0.01) as compared to controls.
Conclusion:
We found that NKTR-255 significantly enhanced the ADCC of expanded NK cells with anti-CD20 type I and type II antibodies against CLL, FL and rituximab-resistant BL cells in vitro with enhanced IFN-g, granzyme B and perforin release. The in vivo effects of NKTR-255 with expanded NK cells and anti-CD20 type I and type II antibodies against CLL, FL and rituximab-resistant BL cells using humanized NSG models are under investigation.
Lee:Kiadis Pharma Netherlands B.V: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Madakamutil:Nektar Therapeutics: Current Employment. Marcondes:Nektar Therapeutics: Current Employment. Klein:Roche: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Cairo:Nektar Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Miltenyi: Research Funding; Technology Inc/Miltenyi Biotec: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal